Overview
Problem
Pfizer managed two web properties:
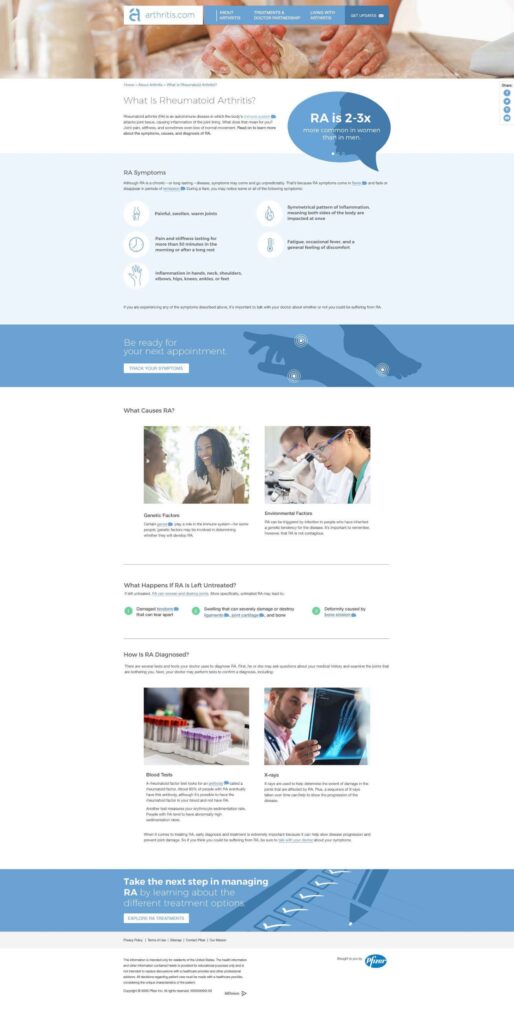
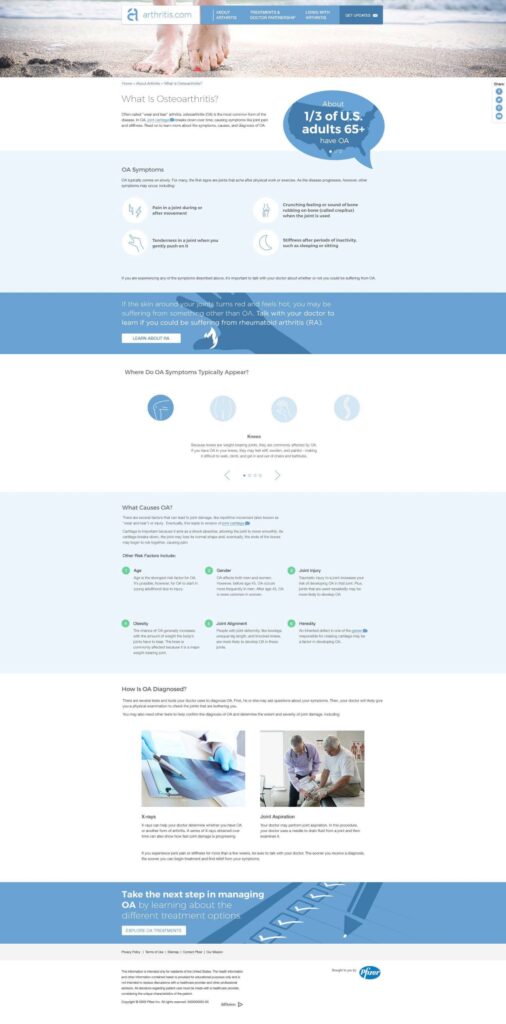
- arthritis.com: This was an outdated, unbranded site offering basic information on rheumatoid arthritis. Despite its dated design, the site saw significant organic traffic, a low bounce rate, and high on-site engagement. However, there was minimal promotion of Pfizer's branded property, with only a single banner on an internal page driving traffic to it.
- rethinkra.com: Another unbranded site, this one focused on providing an unbranded support kit.
The client tasked us with rebranding arthritis.com, integrating rethinkra.com into the new site, adding lifestyle content, featuring key influencers in the field, and implementing a cohesive linking strategy. This approach aimed to capitalize on the existing traffic and better expose the audience to the range of resources Pfizer could offer, from disease education to progress trackers.
Outcome
The project was divided into four phases to enable faster delivery and streamline work streams. The final merged website was fully compliant with the FDA’s Fair Balance guidance while featuring high-quality content from lifestyle influencers in the rheumatoid arthritis (RA) space:
- A unified online platform allowed for a more efficient media buy.
- Lifestyle-focused, unbranded content boosted user engagement.
- An unbranded email campaign maintained continuous touchpoints with the qualified audience.
- The responsive website removed accessibility barriers, improving usability.
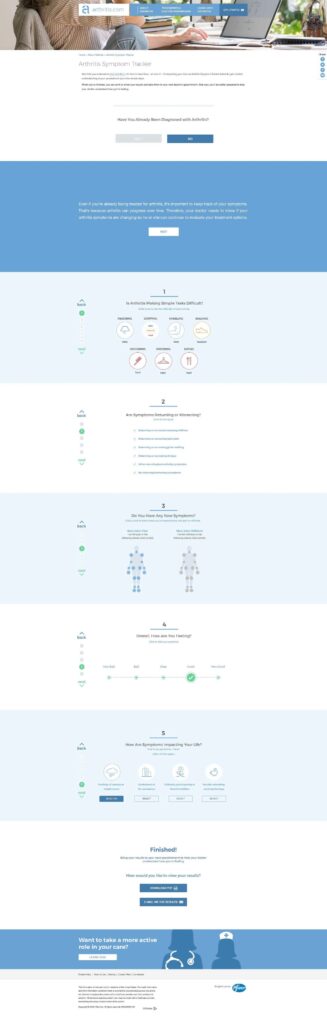
- A suite of interactive tools reinforced Pfizer’s commitment to supporting every patient.
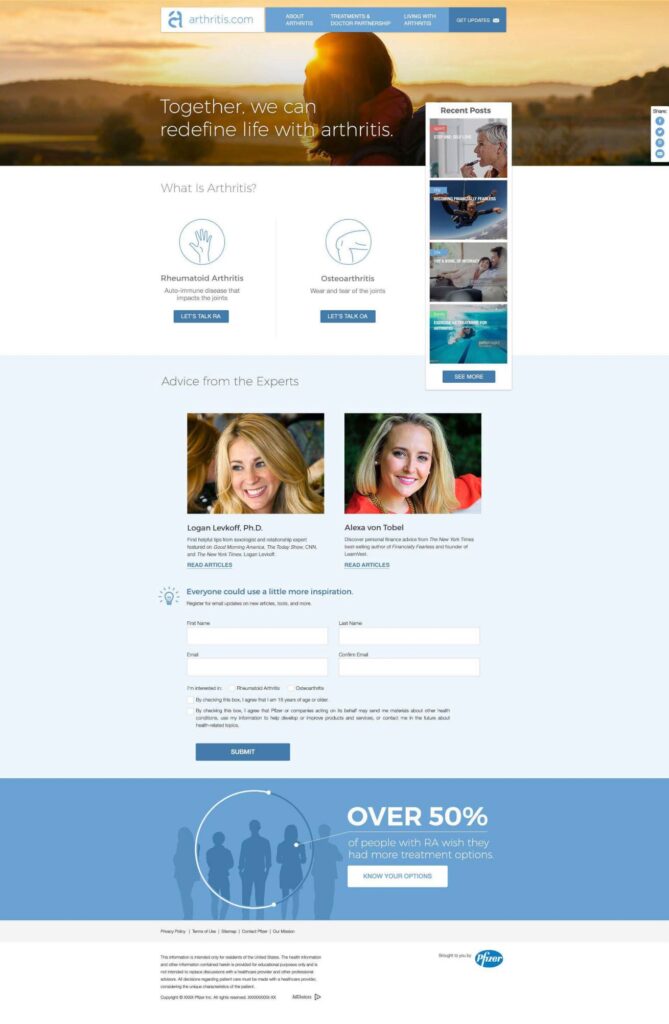
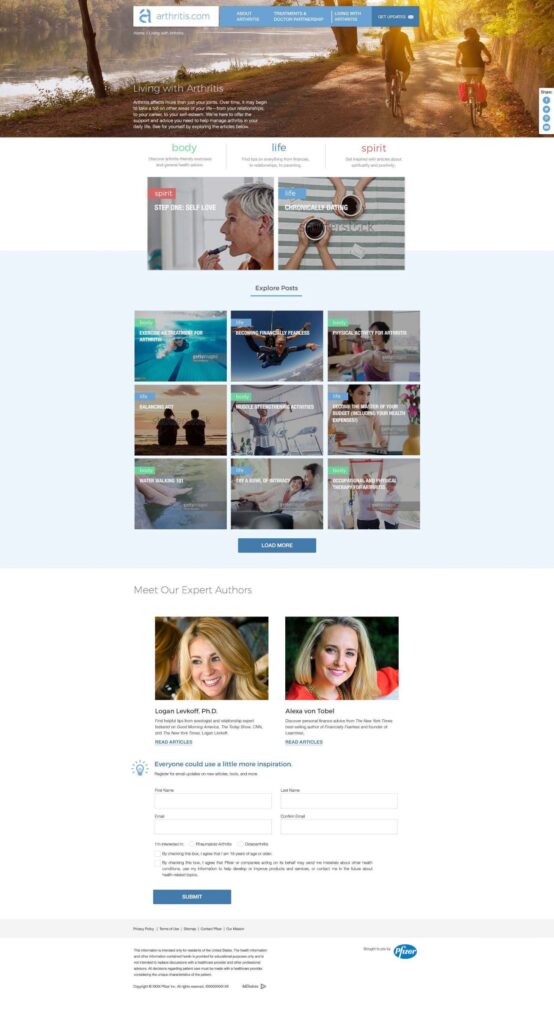
- All content on arthritis.com was centered around three key themes: education, empowerment, and support.
Company
Pfizer
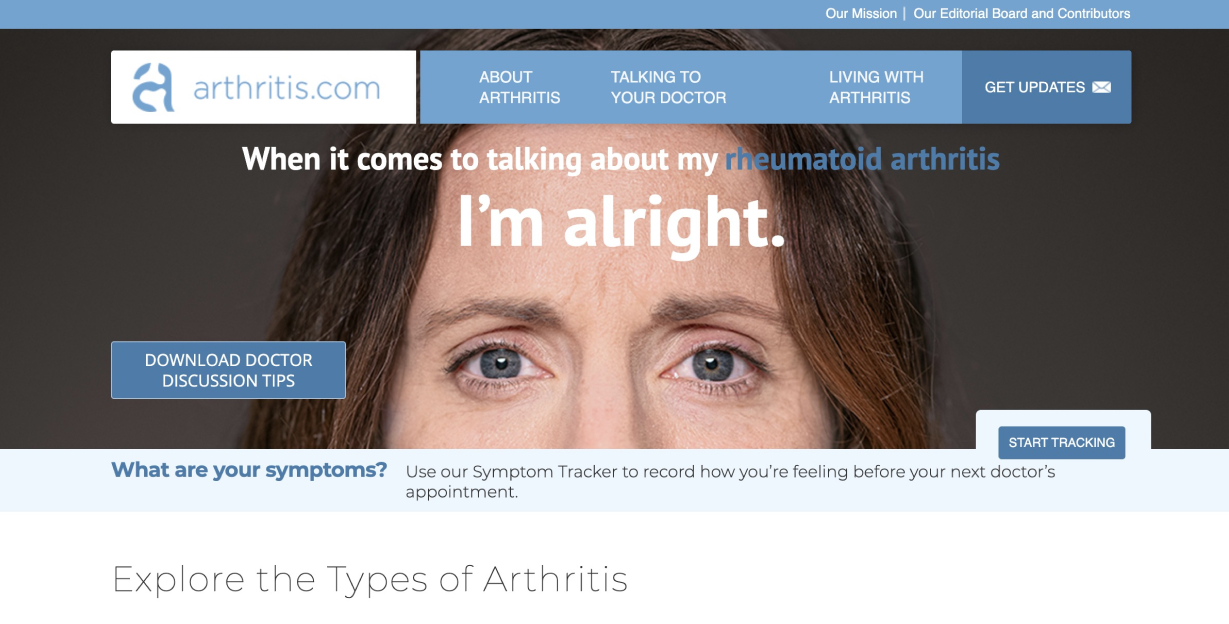
Arthirits.com has a mission to help patients take control of life with arthritis by creating a home for arthritis education, empowerment, and support.
Arthritis.com is an unbranded website with a focus on patient empowerment via condition and lifestyle content. The site caters to both naïve and experienced audiences with the main aim of producing qualifying leads and feeding branded CRM for Xeljanz, a small pill that treats moderate to severe rheumatoid arthritis. Registration is a key component of connecting unbranded and branded CRM efforts by Pfizer within Xeljanz ecosystem.
Info
Role
Senior UX Designer
Team
Copywriter, UI Designer, Data Analyst, PM, Account
Engagement Length
2 Year
Tools
Sketch, Photoshop
Understanding the problem
Why are we doing this?
1
Business Side
Empower patients to engage in a proactive dialogue with their Health Care Providers.
Improve broad awareness of xeljanz.com to ensure patients understand the total value proposition.
Merge web properties to ensure efficient paid media spend.
Become a trusted source for all arthritis education.
_
2
Customer Side
Patients are overwhelmed by the volume of information and resources and have trouble finding answers to specific questions – especially at the point of diagnosis.
Patients also have difficulty communicating with loved ones and health care professionals about their disease as it is considered to be a "hidden" disease.
Patients also lack life-style advise that could help alleviate their symptoms and help them copy with their disease better.
Approach
Discovery
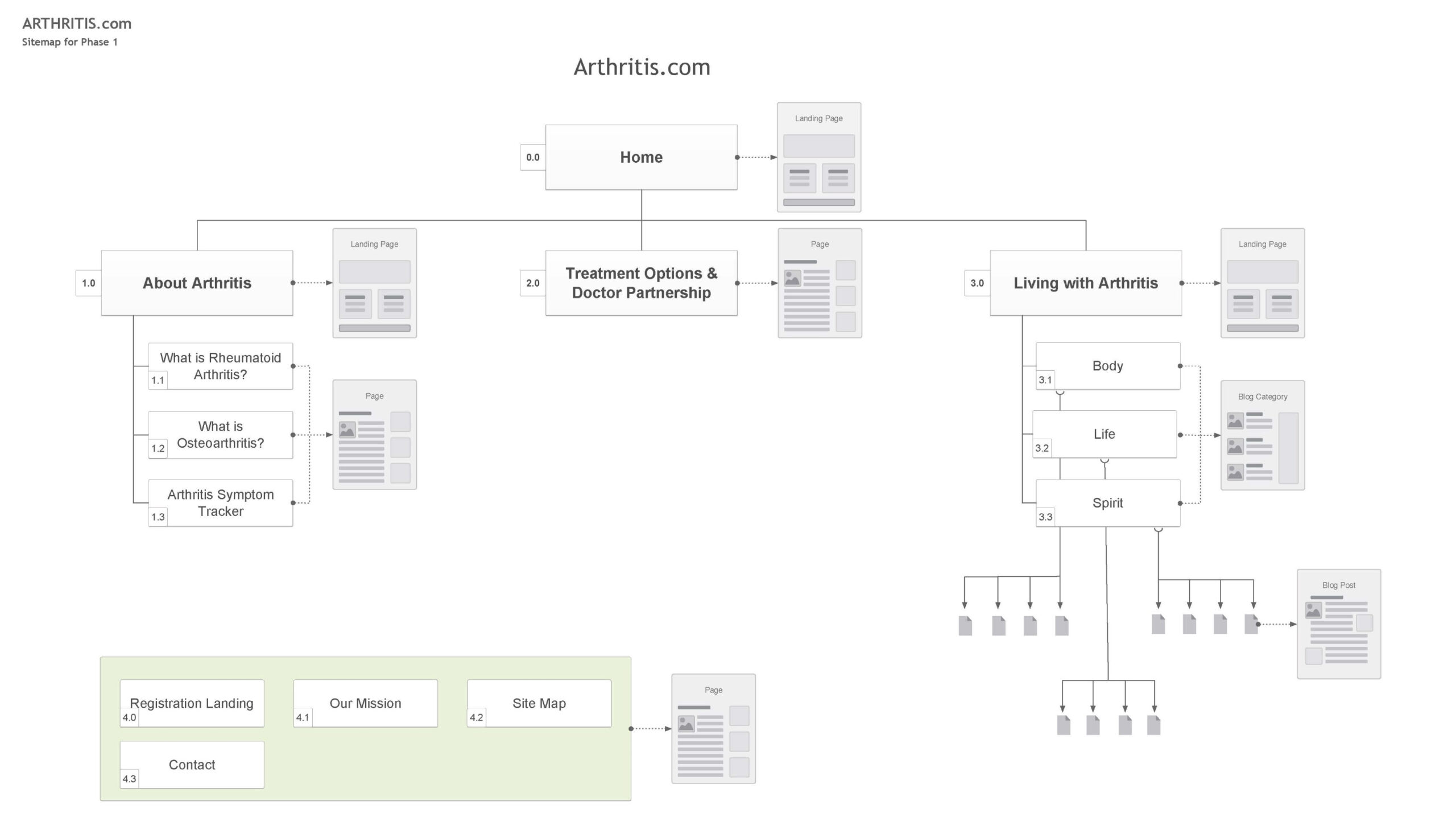
Content Map for Phase 1

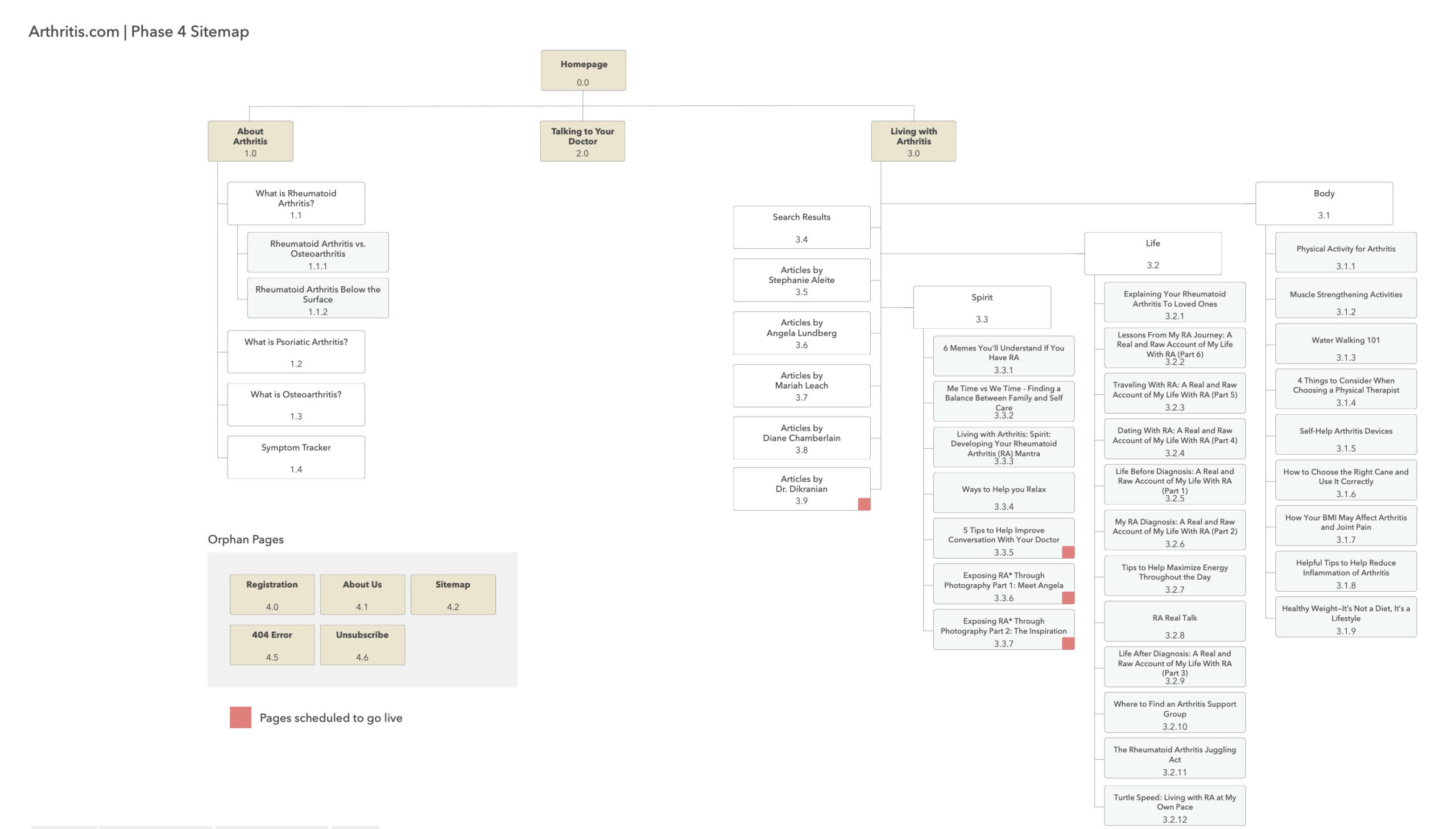
Information Architecture for Phase 1 and Phase 4
Audience Matters
Client and Regulatory Approvals
All materials prepared for distribution must undergo evaluation by Pfizer's Review Committee (RC) to ensure factual accuracy and adherence to applicable laws, regulations, and Pfizer policies.
While client reviews may take place in a clickable prototype—such as an inVision prototype—a website must ultimately be packaged as a PDF. This means all text and images must be placed in the final layout, internally approved, reviewed by the client, and then presented.
Errors can be costly, leading to delays, revisions, and extended timelines.
Wireframing
Mobile first approach
Client approval of the sitemap enabled me to efficiently organize the content, identify areas requiring new content, and update existing, RC-approved content.
Wherever possible, I used real content to avoid complications with the RC review process.
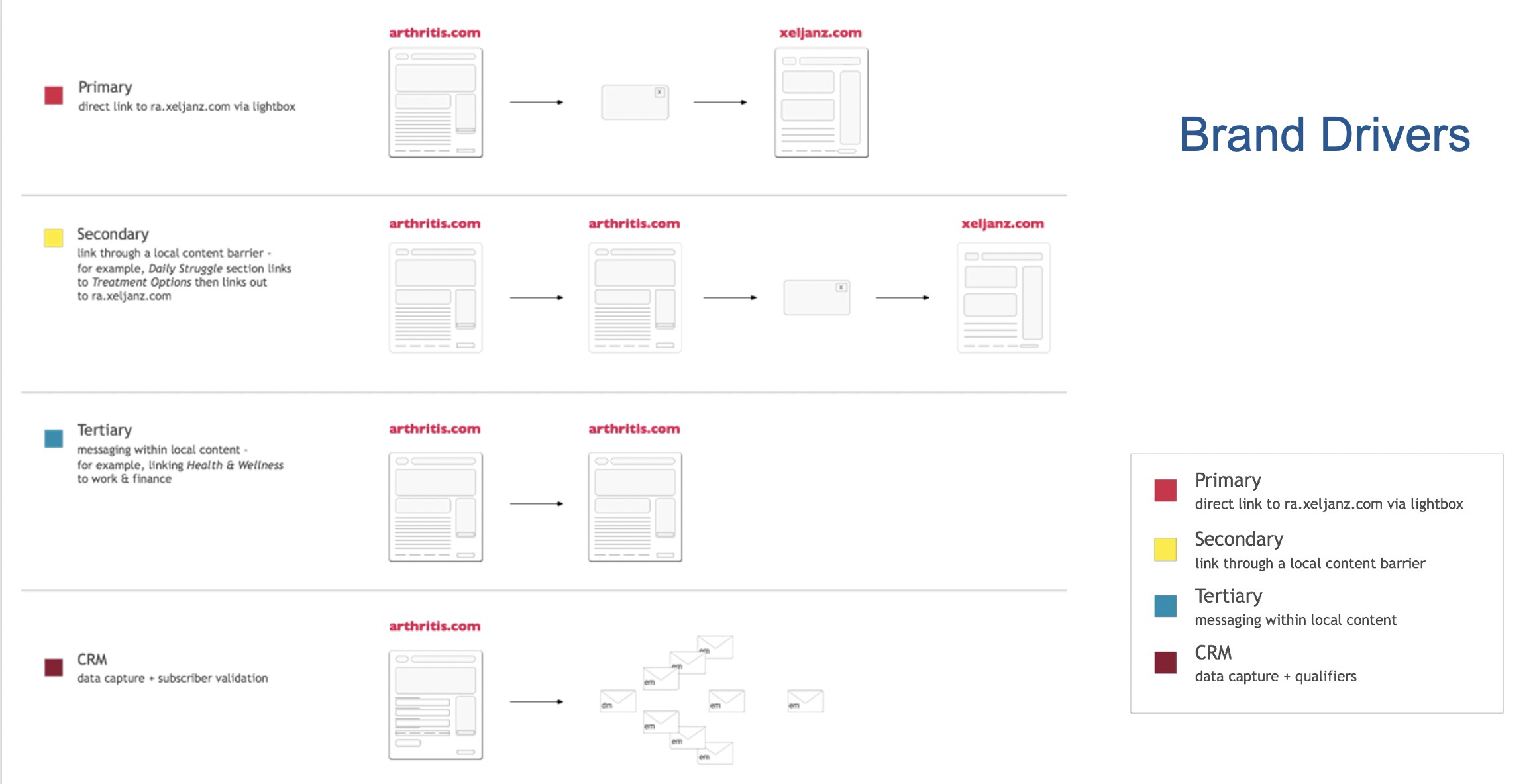
I began by developing a complete set of wireframes for mobile. Additionally, I created a map of brand drivers to ensure that the copy, design, and strategy worked together seamlessly, establishing a cohesive linking strategy for the website.
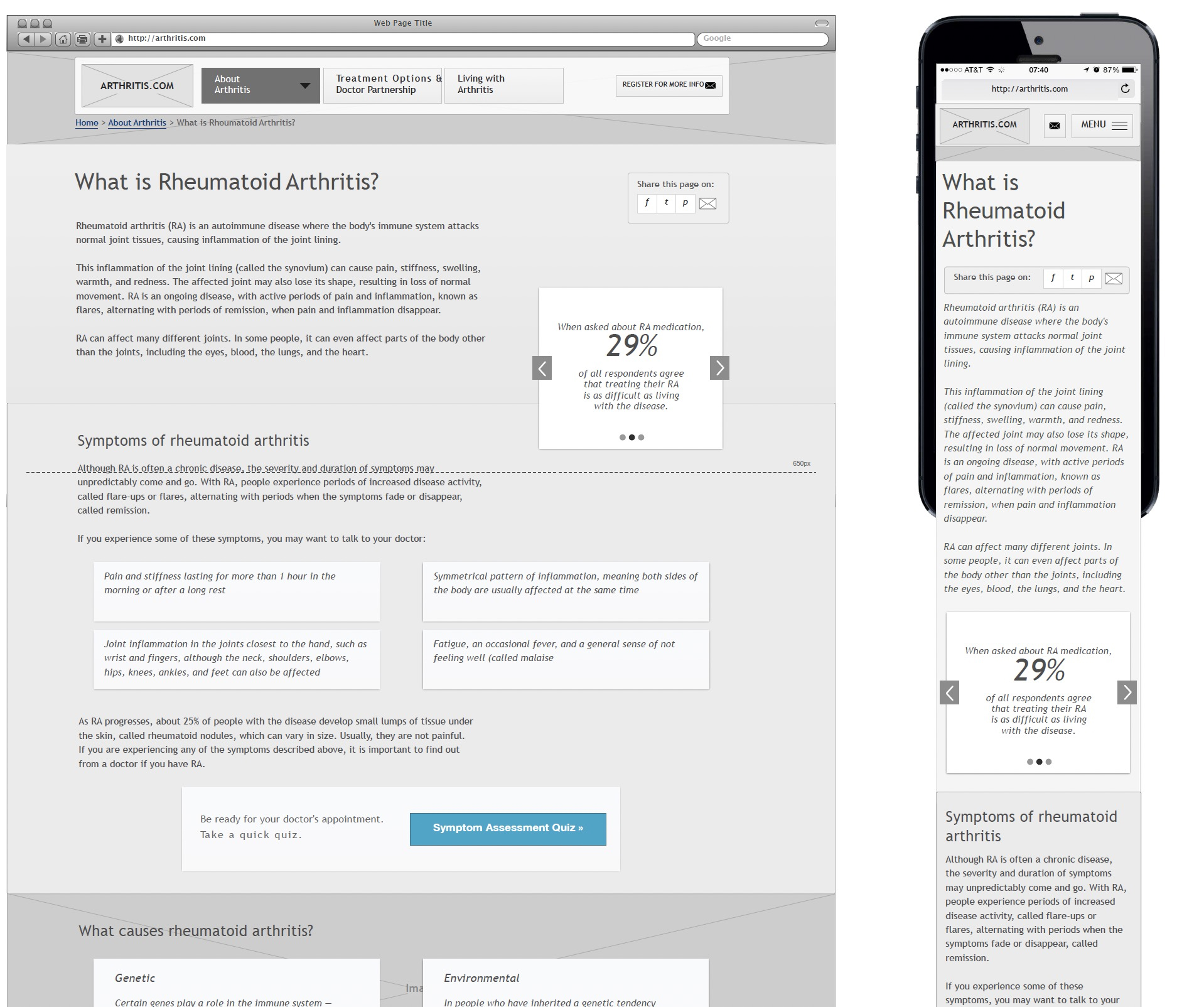
Design Process
Working with Design
Once the full set of wireframes was delivered to the design team, I streamlined all components to ensure consistency, with a focus on simplifying development and future maintenance.
Development Handoff
From PDF to JIRA
Historically, the hand-offs were done via annotated PDFs leading to numerous hours wasted in meetings trying to find a shared understanding of the goals.
I've championed using JIRA as a primary tool for the hand-off to create yet another efficiency. I mapped all the global elements and main pages to Epics, while using Stories for details.
JIRA also allowed for better QA, leading to minimizing miscommunication and better direction for the off-shore development team.
Tracking
Measuring the success
An additional advancement was the integration of Hotjar as a primary tool for real-time, 'in-the-wild' tracking across our entire user base. User data was anonymized in strict compliance with Pfizer's privacy guidelines.
This approach, along with Google Analytics, increased visits quarter-over-quarter. Engagement on mobile devices exploded as the website was fully responsive.
The website saw a decrease in bounce traffic from 60% to 33% YoY, a well as an increased duration of the visit from 4 to 6min